Acylsilane directed aromatic C–H alkenylations by ruthenium catalysis†
Abstract
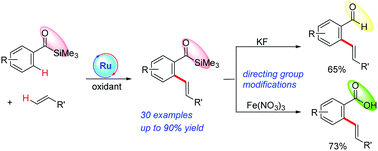
A ruthenium-catalyzed C–H alkenylation of aroylsilanes with electron-deficient alkenes was developed, using acylsilane as the directing group. The mild reaction conditions enable the tolerance of a wide scope of functionalities such as OMe, F, Cl, Br and CF3, providing a convenient and highly effective method for the synthesis of styrene derivatives bearing acylsilane. Steroid and heterocycles such as furan and thiophene were also well tolerated. Furthermore, acylsilane was efficiently converted to the corresponding aldehyde or carboxylic acid.


 Please wait while we load your content...
Please wait while we load your content...