Biocatalytic selective functionalisation of alkenes via single-step and one-pot multi-step reactions
Abstract
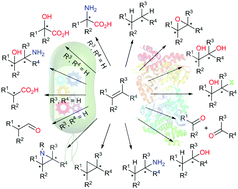
Alkenes are excellent starting materials for organic synthesis due to the versatile reactivity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and the easy availability of many unfunctionalised alkenes. Direct regio- and/or enantioselective conversion of alkenes into functionalised (chiral) compounds has enormous potential for industrial applications, and thus has attracted the attention of researchers for extensive development using chemo-catalysis over the past few years. On the other hand, many enzymes have also been employed for conversion of alkenes in a highly selective and much greener manner to offer valuable products. Herein, we review recent advances in seven well-known types of biocatalytic conversion of alkenes. Remarkably, recent mechanism-guided directed evolution and enzyme cascades have enabled the development of seven novel types of single-step and one-pot multi-step functionalisation of alkenes, some of which are even unattainable via chemo-catalysis. These new reactions are particularly highlighted in this feature article. Overall, we present an ever-expanding enzyme toolbox for various alkene functionalisations inspiring further research in this fast-developing theme.
C bonds and the easy availability of many unfunctionalised alkenes. Direct regio- and/or enantioselective conversion of alkenes into functionalised (chiral) compounds has enormous potential for industrial applications, and thus has attracted the attention of researchers for extensive development using chemo-catalysis over the past few years. On the other hand, many enzymes have also been employed for conversion of alkenes in a highly selective and much greener manner to offer valuable products. Herein, we review recent advances in seven well-known types of biocatalytic conversion of alkenes. Remarkably, recent mechanism-guided directed evolution and enzyme cascades have enabled the development of seven novel types of single-step and one-pot multi-step functionalisation of alkenes, some of which are even unattainable via chemo-catalysis. These new reactions are particularly highlighted in this feature article. Overall, we present an ever-expanding enzyme toolbox for various alkene functionalisations inspiring further research in this fast-developing theme.


 Please wait while we load your content...
Please wait while we load your content...