Annulating thiazolium cations via a direct double C–H activation strategy: Rh–N,S-heterocyclic carbene is the key†
Abstract
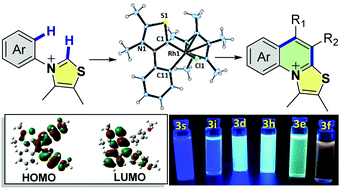
Herein we show how metal–N,S-heterocyclic carbene intermediates are conveniently generated and utilized for the first time to construct N,S doubly-doped cationic conjugated tricyclic organic molecules which exhibit tuneable emission. The chemistry proceeds via direct double C–H activation followed by an annulative π-extension pathway.


 Please wait while we load your content...
Please wait while we load your content...