Stability of doubly and triply H-bonded complexes governed by acidity–basicity relationships†
Abstract
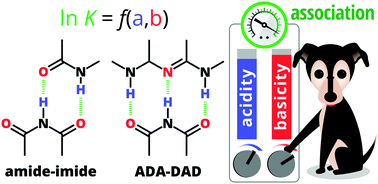
We used our recently proposed acidity–basicity interplay (ABI) model (Chem. Sci., 2018, 9, 4402) and the Jorgensen secondary interactions hypothesis (JSIH) to rationalise the experimentally observed trends in the formation constants of doubly and triply H-bonded systems with –NH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– and –NH⋯N– interactions. Unlike the JSIH, the ABI interpretation can explain the trends in the complexation of amide/imide homo- and heterodimers as well as ADA–DAD clusters. We found that the strongest H-bonds play a very important role, a condition which offers an alternative to the well established JSIH to modulate the stability of these relevant systems.
C– and –NH⋯N– interactions. Unlike the JSIH, the ABI interpretation can explain the trends in the complexation of amide/imide homo- and heterodimers as well as ADA–DAD clusters. We found that the strongest H-bonds play a very important role, a condition which offers an alternative to the well established JSIH to modulate the stability of these relevant systems.


 Please wait while we load your content...
Please wait while we load your content...