Self-assembled multivalent (SAMul) ligand systems with enhanced stability in the presence of human serum†
Abstract
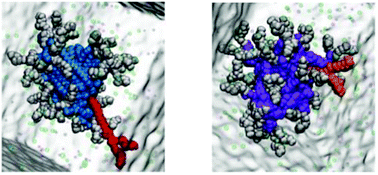
Self-assembled cationic micelles are an attractive platform for binding biologically-relevant polyanions such as heparin. This has potential applications in coagulation control, where a synthetic heparin rescue agent could be a useful replacement for protamine, which is in current clinical use. However, micelles can have low stability in human serum and unacceptable toxicity profiles. This paper reports the optimisation of self-assembled multivalent (SAMul) arrays of amphiphilic ligands to bind heparin in competitive conditions. Specifically, modification of the hydrophobic unit kinetically stabilises the self-assembled nanostructures, preventing loss of binding ability in the presence of human serum – cholesterol hydrophobic units significantly outperform systems with a simple aliphatic chain. It is demonstrated that serum albumin disrupts the binding thermodynamics of the latter system. Molecular simulation shows aliphatic lipids can more easily be removed from the self-assembled nanostructures than the cholesterol analogues. This agrees with the experimental observation that the cholesterol-based systems undergo slower disassembly and subsequent degradation via ester hydrolysis. Furthermore, by stabilising the SAMul nanostructures, toxicity towards human cells is decreased and biocompatibility enhanced, with markedly improved survival of human hepatoblastoma cells in an MTT assay.


 Please wait while we load your content...
Please wait while we load your content...