Amphiphilic tri- and tetra-block co-polymers combining versatile functionality with facile assembly into cytocompatible nanoparticles†
Abstract
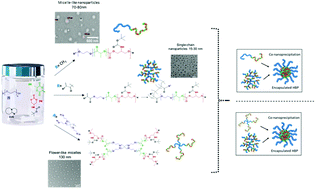
In order for synthetic polymers to find widespread practical application as biomaterials, their syntheses must be easy to perform, utilising freely available building blocks, and should generate products which have no adverse effects on cells or tissue. In addition, it is highly desirable that the synthesis platform for the biomaterials can be adapted to generate polymers with a range of physical properties and macromolecular architectures, and with multiple functional handles to allow derivatisation with ‘actives’ for sensing or therapy. Here we describe the syntheses of amphiphilic tri- and tetra-block copolymers, using diazabicyclo[5.4.0]undec-5-ene (DBU) as a metal-free catalyst for ring-opening polymerisations of the widely-utilised monomer lactide combined with a functionalised protected cyclic carbonate. These syntheses employed PEGylated macroinitiators with varying chain lengths and architectures, as well as a labile-ester methacrylate initiator, and produced block copolymers with good control over monomer incorporation, molar masses, side-chain and terminal functionality and physico-chemical properties. Regardless of the nature of the initiators, the fidelity of the hydroxyl end group was maintained as confirmed by a second ROP chain extension step, and polymers with acryloyl/methacryloyl termini were able to undergo a second tandem reaction step, in particular thiol–ene click and RAFT polymerisations for the production of hyperbranched materials. Furthermore, the polymer side-chain functionalities could be easily deprotected to yield an active amine which could be subsequently coupled to a drug molecule in good yields. The resultant amphiphilic copolymers formed a range of unimolecular or kinetically-trapped micellar-like nanoparticles in aqueous environments, and the non-cationic polymers were all well-tolerated by MCF-7 breast cancer cells. The rapid and facile route to such highly adaptable polymers, as demonstrated here, offers promise for a range of bio materials applications.
- This article is part of the themed collection: 29th Annual Conference of the European Society for Biomaterials


 Please wait while we load your content...
Please wait while we load your content...