Self-assembled nano-aggregates of fluorinases demonstrate enhanced enzymatic activity, thermostability and reusability
Abstract
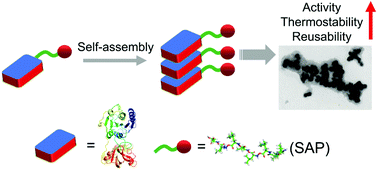
Three SAP (self-assembling peptide)-tagged fluorinases (FLAs), namely, FLA-ELK16, FLA-L6KD and FLA-18A (named after the SAP used for tagging FLA) were successfully engineered. All three SAP-tagged FLAs could be highly over-expressed using engineered E. coli host cells despite being in the form of aggregates (inclusion bodies). It was noted that all three SAP-tagged FLAs exhibited enzymatic activity. It was also observed that all three SAP-tagged FLAs were capable of self-assembly to form nano-sized particles with different dimensions in aqueous solutions. Strikingly, one of the SAP-tagged FLA (FLA-L6KD) displayed improved enzyme activity, thermostability and reusability, which is potentially ideal for bio-transformation. FLA is an exotic enzyme that is capable of catalysing the formation of C–F bonds using inorganic fluorine ions as substrates. This significant feature enables it to incorporate [18F]-fluoride into different small molecules to generate radiopharmaceuticals in PET (positron emission tomography) labeling. In addition, fluorinase is greatly valuable in synthetic biology for incorporating the fluorine element into building blocks to produce non-natural organofluorines or as a biocatalyst for transforming non-native substrates. Our method would be a further step in making FLA-based biocatalysis even ‘greener’ by enhancing the enzymatic activity, thermostability and reusability of FLA through the introduction of nano-sized aggregates. Enzymes are such nontrivial biomaterials, which can be manifested in different scenarios. Our research expands their reach and tunes their properties by tagging SAP partners. Thus, this methodology can be put into the ‘toolbox’ of enzymologists, which can be further explored and generalised for others.


 Please wait while we load your content...
Please wait while we load your content...