A progressively targeted gene delivery system with a pH triggered surface charge-switching ability to drive angiogenesis in vivo†
Abstract
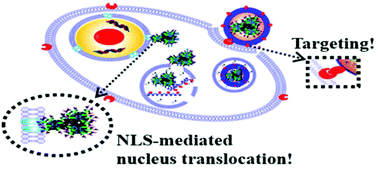
For clinical application of therapeutic gene delivery, it is urgent to develop safe and in vivo efficient delivery systems. Nowadays, gene delivery carriers based on functional peptides have attracted much attention due to their excellent biocompatibility, biodegradability and biological multifunctionality. In the present study, a star-shaped integrated functional peptide, polyhedral oligomeric silsesquioxane (POSS-(C-G-NLS-G-TAT)16, abbreviated as PP1), was synthesized through “thiol–ene” click chemistry between the TAT-G-NLS-G-C multifunctional peptide sequence and inorganic octa-diallyl POSS. Cationic PP1 was mixed with the pZNF580 plasmid to obtain stable binary gene complexes (BCPs) with membrane penetrating and nucleus targeting functions. In order to improve BCPs’ biocompatibility, cellular uptake, and endosome escape, they were further modified using an anionic polymer of PLL-g-CAGW21%-g-Acon (n = 47%, 57% and 64%) having an EC targeting ligand (CAGW peptide) and a charge reversal moiety (cis-aconitic amide) through electrostatic absorption to obtain ternary gene complexes (TCPs). By adjusting the weight ratio of PP1/pZNF580 plasmid/PLL-g-CAGW21%-g-Acon to 5/1/1.25, TCPs-1 with n = 47%, TCPs-2 with n = 57% and TCPs-3 with n = 64% exhibited a neutral zeta potential and suitable particle size; thus they were used for further biological evaluation. Compared with BCPs (5/1 weight ratio of PP1/pZNF580 plasmid), TCPs exhibited high hemocompatibility and cytocompatibility; more interestingly, they also showed significantly enhanced gene delivery efficiency. The TCP groups achieved perfect transfection effects in the proliferation and migration of human umbilical vein endothelial cells (HUVECs), and especially high neovascularization in vitro and in vivo. Our results demonstrated that the high graft ratio of cis-aconitic amide provided benefits of high biocompatibility and gene delivery efficiency, and the TCPs-3 group showed the optimized transfection efficiency among the three groups. Importantly, HUVECs transfected with TCPs-3 exhibited an outstanding ability to enhance angiogenesis in vivo. In brief, this multifunctional ternary gene system with the EC targeting ligand and membrane penetrating, charge reversal and nucleus targeting functions is a promising platform for the transfection of HUVECs, and may be useful for the treatment of cardiovascular diseases in clinical applications.


 Please wait while we load your content...
Please wait while we load your content...