A chimeric, multivalent assembly of galectin-1 and galectin-3 with enhanced extracellular activity†
Abstract
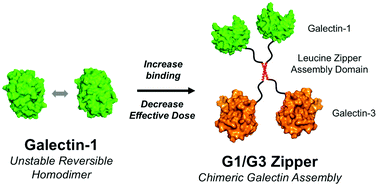
Galectins are attractive therapeutic candidates to control aberrant immune system activation because they can alter the phenotype and function of various innate and adaptive immune cells. However, use of exogenous galectin-1 (“G1”) and galectin-3 (“G3”) as immunomodulators is challenged by their high dosing requirements and dynamic quaternary structures. Here we report a chimeric assembly of G1 and G3 with enhanced extracellular activity (“G1/G3 Zipper”), which was created by recombinant fusion of G1 and G3 via a peptide linker that forms a two-stranded α-helical coiled-coil. G1/G3 Zipper had higher apparent binding affinity for immobilized lactose and a lower concentration threshold for inducing soluble glycoprotein crosslinking than G1, a recombinant fusion of G1 and G3 with a flexible peptide linker (“G1/G3”), or a recently reported stable G1 dimer crosslinked by poly(ethylene glycol) diacrylate (“G1-PEG-G1”). As a result, G1/G3 Zipper was more effective at inducing Jurkat T cell apoptosis in media containing serum, and was the only variant that could induce apoptosis at low concentrations under serum-free conditions. The monomeric G1/G3 fusion protein lacked extracellular activity under all conditions tested, suggesting that the enhanced activity of G1/G3 Zipper was due to its quaternary structure and increased carbohydrate-recognition domain valency. Thus, combining G1 and G3 into a non-native chimeric assembly provides a new candidate therapeutic with greater immunomodulatory potency than the wild-type proteins and previously reported engineered variants.
- This article is part of the themed collection: Biomaterial Interactions with the Immune System


 Please wait while we load your content...
Please wait while we load your content...