A near infrared-modulated thermosensitive hydrogel for stabilization of indocyanine green and combinatorial anticancer phototherapy†
Abstract
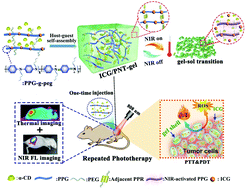
Indocyanine green (ICG), a multifunctional near-infrared (NIR) imaging agent approved by the FDA, has been extensively used in clinical cancer theranosis, but limited by its inherent instability, short plasma half-life and lack of targeting ability. Herein, an in situ formed photothermal network based thermosensitive hydrogel (PNT-gel) constructed by using supramolecular cross-linking conjugated polymers was developed for the stabilization of ICG and efficient combinatorial photothermal/photodynamic antitumor therapy. While the conjugated polymeric backbone in PNT-gel anchored the aromatic phototherapeutic agent ICG via π–π stacking interactions to avoid premature leakage, it also directly converted low-dose NIR light to induce localized hyperthermia to enhance the photothermal effect. The PNT-gel shows a reversible gel-to-sol upper critical solution temperature (UCST) that is slightly above body temperature. Therefore, the controlled release of ICG was switched on or off by NIR via photothermal-induced gel–sol transition. In vitro and in vivo antitumor experiments demonstrated that ICG loaded PNT-gel not only efficiently induced the killing of 4T1 cancer cells, but also achieved almost complete eradication of 4T1 cells by one-dose intratumoral injection in combinatorial photothermal/photodynamic therapy under irradiation of a low-dose 808 nm laser (0.14 W cm−2). Additionally, the combinational therapy proved to enhance the effectiveness of photodestruction without tumor recurrence compared with photothermal therapy (PTT) or photodynamic therapy (PDT) treatment alone.


 Please wait while we load your content...
Please wait while we load your content...