Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization
Abstract
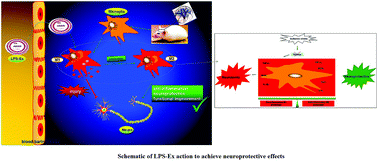
Inflammation occurs throughout the progression of cerebral ischemia/reperfusion and mediates myriads of pathological events following an ischemic insult. After ischemic stroke, inflammation was significantly triggered and microglia were activated to phenotypes (M1) typically releasing pro-inflammatory mediators, thus inducing neuron apoptosis and exacerbating brain injury. Therefore, shifting the polarization of microglia from the detrimental pro-inflammatory M1 phenotype to the beneficial anti-inflammatory M2 phenotype seems a promising therapeutic strategy in ischemic stroke. In this study we aimed to investigate the effects of exosomes (Ex) secreted from the lipopolysaccharide (LPS)-stimulated macrophage RAW264.7 cell line (LPS-Ex) on inducing neuroprotection and functional improvement after ischemic stroke by enhancing microglial M2 polarization. The results showed that LPS-Ex treatment exhibited more potent anti-inflammatory and neuroprotective effects in vitro; furthermore it significantly reduced the brain infarct volume and improved neurological function in a rat model of transient focal cerebral ischemia. The beneficial effects of LPS-Ex in ischemic stroke were associated with enhancing microglial polarization from the M1 phenotype to the M2 phenotype and inhibiting inflammation response. In conclusion, LPS-Ex protected against cerebral ischemia by skewing the microglial functional polarity from M1 toward an anti-inflammatory M2 phenotype. It may serve as a novel therapeutic strategy for neuroprotection and functional recovery upon ischemic stroke onset.


 Please wait while we load your content...
Please wait while we load your content...