Porous protein crystals as scaffolds for enzyme immobilization†
Abstract
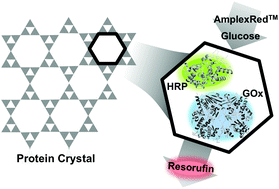
Porous protein crystals provide a template for binding and organizing guest macromolecules. Peroxidase, oxidase, and reductase enzymes immobilized in protein crystals retained activity in single-crystal and bulk assay formats. Several binding strategies, including metal affinity and physical entrapment, were employed to encourage enzyme adsorption into the protein crystals and to retain the enzymes for multiple recycles. Immobilized enzymes had lower activity compared to free enzyme in solution, in part due to diffusion limitations of substrate within the crystal pores. However, the immobilized enzymes were long-term stable and showed increased thermal tolerance. The potential applications of enzyme-laden crystals as sensing devices, delivery capsules, and microreactors motivate future development of this technology.


 Please wait while we load your content...
Please wait while we load your content...
