Red blood cell-derived nanovesicles for safe and efficient macrophage-targeted drug delivery in vivo†
Abstract
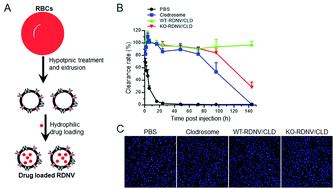
Macrophage-targeted drug delivery has great therapeutic potential for the treatment of cancers and inflammatory diseases. There is also an unmet need for efficient and nontoxic means of in vivo macrophage depletion to determine the role of macrophages under normal and disease settings. Herein, we explored the potential of red blood cell (RBC)-derived nanovesicles (RDNVs) as drug nanocarriers to specifically deplete macrophages. We show that RDNVs are effective hydrophilic drug carriers and can effectively deliver drugs into macrophages both in vitro and in vivo. Nanovesicles derived from both wild-type mouse RBCs (WT-RDNVs) and CD47 KO mouse RBCs (KO-RDNVs) can encapsulate clodronate with good stability in PBS for long-term storage. However, KO-RDNVs were more efficiently engulfed by macrophages in vitro and more rapidly cleared in vivo than WT-RDNVs, indicating that CD47 also serves as a “don't eat me” molecule for RDNVs as it does for RBCs. Accordingly, clodronate-encapsulated KO-RDNVs (KO-RDNV/CLD) were significantly more toxic to mouse macrophages in vitro than drug-loaded WT-RDNVs (WT-RDNV/CLD). Furthermore, WT-RDNV/CLD showed prolonged accumulation in tissues (e.g., liver and lung) and macrophage depletion versus KO-RDNV/CLD. Importantly, RBC-derived nanovesicles are more biocompatible and less toxic in vivo than clodronate-encapsulated liposomes—the current gold-standard macrophage-depleting reagent. This study offers a useful strategy for macrophage-targeted drug delivery.


 Please wait while we load your content...
Please wait while we load your content...