Toward reducing biomaterial antigenic potential: a miniaturized Fc-binding domain for local deposition of antibodies†
Abstract
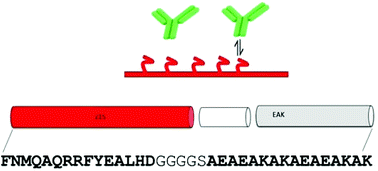
A peptide derived from staphylococcal protein A (SpA) was developed as an affinity module for antibody delivery applications. The miniaturized protein consists of the first helix of the engineered SpA Z domain fused with the self-assembling peptide (SAP) AEAEAKAKAEAEAKAK, or EAK. The resulting peptide, named Z15_EAK, was shown to possess fibrillization properties and an Fc-binding function. The peptide induced a red shift in the Congo red absorbance characteristic of peptide fibrils, also evidenced in transmission electron microscopy images. The one-site binding affinity (Kd) of a gel-like coacervate generated by admixing Z15_EAK with EAK for IgG was determined to be 1.27 ± 0.14 μM based on a microplate-based titration assay. The coacervate was found to localize IgG subcutaneously in mouse footpads for 8 to 28 days. A set of in vivo data was fit to a one-compartment model for simulating the relative fractions of IgG dissociated from the materials in the depot. The model predicted that close to 27% of the antibodies injected were available unbound for the duration of the experiment. Z15_EAK did not appear to induce innate immune responses; injecting Z15_EAK into mouse footpads elicited neither interleukin-6 (IL-6) nor tumor necrosis factor-alpha (TNF-α) from splenocytes isolated from the animals one day, seven days, or eleven days afterward. The antigenic potential of Z15 was analyzed using a bioinformatic approach in predicting sequences in SpA and Z15 dually presented by class I and class II human MHC alleles covering the majority of the population. A peptide in SpA identified as a potential T cell epitope cross reacting with a known epitope in a microbial antigen was eliminated by miniaturization. These results demonstrate that Z15_EAK is a potential platform for generating antibody depots by which the impacts of Fc-based biotherapeutics can be enhanced through spatiotemporal control.
- This article is part of the themed collection: Biomaterial Interactions with the Immune System


 Please wait while we load your content...
Please wait while we load your content...
