Determination of residual phenylhydrazines in drug substances by high-performance liquid chromatography with pre-column derivatization
Abstract
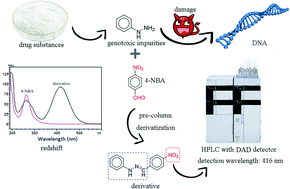
Phenylhydrazines, which are important intermediates in pharmaceutical synthesis, are known as genotoxic or mutagenic impurities. They are only allowed to be present at trace levels in drug products and therefore their control is critical in pharmaceutical development. A conventional high-performance liquid chromatograph with an ultraviolet (UV) detector is always used for the test of phenylhydrazines in drugs, but the similar maximum UV absorption wavelengths of phenylhydrazines and the drug matrix or related substances make the method lack specificity. In this paper, a simple pre-column derivatization approach was applied to selectively move the maximum absorption wavelengths of residual phenylhydrazines in drug substances to the visible region, in which most drugs have little absorption. Phenylhydrazine was chosen as the model analyte for the establishment and validation of the method. Different derivatization reagents were systematically compared, and 4-nitrobenzaldehyde was finally selected as the most suitable choice. This was because the maximum absorption wavelength of its derivative had the largest redshift to 416 nm with a strong absorption intensity, which could not only reduce the matrix interference from drug substances and related substances, but also largely minimize the interference of the derivatization reagent itself. After optimizing the reaction conditions, the limits of detection and quantification were 0.008 μg mL−1 and 0.02 μg mL−1, respectively. This method was successfully applied for the determination of residual phenylhydrazine in antipyrine and indapamide, and 4-hydrazinylbenzenesulfonamide in celecoxib.


 Please wait while we load your content...
Please wait while we load your content...