High-throughput amperometric determination of tetracycline residues in milk and quality control of pharmaceutical formulations: flow-injection versus batch-injection analysis
Abstract
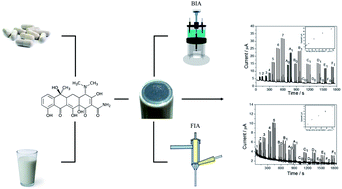
This paper demonstrates a potential use of the reduced graphene-oxide (rGO) modified electrode associated with FIA and BIA systems for rapid, simple and sensitive determination of tetracycline residues in milk samples as well as quality control of pharmaceutical formulations. Experiments involving cyclic voltammetry using the rGO-modified electrode evidenced the anticipation of potential and increase in analytical signal (7-fold for the first oxidation process) when compared to the unmodified electrode, which demonstrated the electrocatalytic effect of rGO responsible for the improved selectivity of the sensor. High- or low-fat milk samples were only diluted in a supporting electrolyte (BR buffer solution) while pharmaceutical tablets were powdered and dissolved in the same electrolyte, and no interferences from the sample matrix were verified in the amperometric determination of tetracycline. Both analytical methods showed high precision (RSD < 3%), low detection limit (1.17 μmol L−1 for FIA and 0.038 μmol L−1 for BIA), high sample throughput (103 h−1), proper accuracy evaluated using recovery values (84–117%) and by comparison with Ultra-Fast Liquid-Chromatography (UFLC) obtaining statistically similar results (95% confidence level). The BIA method presented superior performance regarding sample throughput and especially detection limit due to the high flow injection rate of the system, which is mandatory for the analysis of tetracycline residues in milk. The rGO-modified electrode was stable, sensitive and precise under high-flow conditions even in the presence of high-fat milk samples.
- This article is part of the themed collection: Celebrating Latin American Talent in Chemistry


 Please wait while we load your content...
Please wait while we load your content...