Simultaneous quantification of the CYP2D6 substrate yohimbine, its metabolite 11-OH-yohimbine, and the CYP2D6 inhibitor paroxetine in human plasma†
Abstract
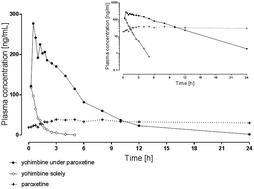
We developed and validated a human plasma LC-MS/MS assay according to FDA guidelines to determine the impact of the CYP2D6 inhibitor paroxetine on the pharmacokinetics of the assumed specific CYP2D6 substrate yohimbine. Yohimbine, its main metabolite 11-OH-yohimbine, and paroxetine were quantified using plasma (100 μL) and liquid–liquid extraction for sample preparation. Analytes were separated with a Phenomenex Luna C18 3 μm LC column using a gradient consisting of ammonium acetate (5 mM), acetic acid, and acetonitrile. Tandem mass spectrometry detection was performed using positive electrospray ionization and selective reaction monitoring utilizing 13C- and deuterium-labeled internal standards in the calibration range from 0.5 to 500 ng mL−1. Accuracy at the lower limit of quantification of 0.5 ng mL−1 was <19% with the corresponding precision being <16%. Within-batch and batch-to-batch accuracies were <14% with the corresponding precision being <12%. Extraction recoveries ranged between 75 and 113% for all analytes. This assay was used to simultaneously quantify plasma concentrations of yohimbine, its metabolite, and paroxetine after oral administration of 5 mg yohimbine solely and in combination with a three-day intake of 20 mg paroxetine to a healthy individual. This enabled the investigation of yohimbine pharmacokinetics and its CYP2D6 dependent metabolization in correlation with plasma exposure to the CYP2D6 inhibitor paroxetine, resulting in doubled maximum concentration, a tenfold increase of AUC and fourfold prolonged elimination half-life.


 Please wait while we load your content...
Please wait while we load your content...