Use of the fluorogenic Al3+–quinolinyl-azo-naphtholato complex for the determination of F− in aqueous medium by visible light excitation and application in ground water fluoride analysis†
Abstract
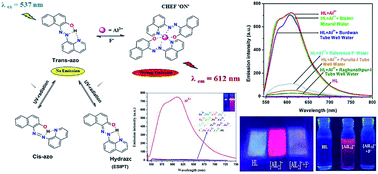
Quinolinyl-azo-naphthol (HL) is a selective turn-on chemosensor for Al3+ in the presence of other ions and the excitation at visible light (537 nm) shows a 750 fold enhancement of emission at 612 nm. The limit of detection (LOD) is 0.69 nM (3σ method), which is far below the WHO recommended value for Al3+ in drinking water (7.41 μM). The composition of the isolated complex [AlL2]+ is supported by ESI-MS spectral data and Job's plot. Upon addition of aqueous F− to the [AlL2]+ solution the emission intensity is quenched and the LOD of F− is 0.63 nM. HL is thus instrumental for the design of portable kits for the detection of fluoride contamination in drinking water. The probe undergoes azo-hydrazo tautomerization and also exhibits an INHIBIT logic gate with Al3+ and F− as chemical inputs by monitoring the emission mode at 612 nm.


 Please wait while we load your content...
Please wait while we load your content...