Reactivity and relative reaction rates of formaldehyde, acetaldehyde, and acetone coexisting with large quantities of acetone on 2,4-dinitrophenylhydrazine-impregnated filters
Abstract
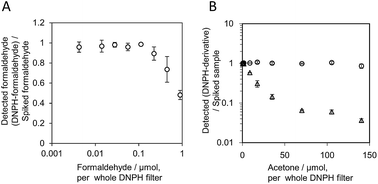
Hazardous substances in the workplace are not always used separately. The reactivity of formaldehyde, acetaldehyde, and acetone on filters impregnated with 2,4-dinitrophenylhydrazine (DNPH) in the presence of large quantities of acetone was analyzed with HPLC and UV-visible spectroscopy to examine the interference of DNPH derivatization caused by coexisting compounds. Formaldehyde (0–0.22 μmol) containing 70 μmol acetone per whole filter was derivatized on a DNPH filter and was found to be affected slightly by acetone. Derivatization of formaldehyde on the DNPH filter occurred much more rapidly than derivatization of acetone. The relative reaction rate was estimated from the quantity of DNPH derivatives and the initial quantities of samples, which was based on what would be expected in an actual workplace formaldehyde sample. The relative reaction rates followed the order formaldehyde > acetaldehyde ≫ acetone. It was confirmed that formaldehyde could be derivatized and quantified in the presence of a large quantity of acetone.


 Please wait while we load your content...
Please wait while we load your content...