Fast determination of omeprazole in extemporaneous suspensions used in paediatrics and stability studies
Abstract
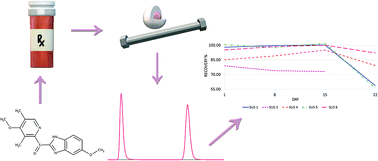
A simple and fast ultra-high-performance liquid chromatography method with UV detection for the separation and quantification of omeprazole and the impurities of omeprazole and methylparaben (the internal standard) in six extemporaneous suspensions has been developed and fully validated. Isocratic elution was carried out using a Kinetex™ C18 column, 50 × 2.1 mm packed with 1.7 μm porous shell particles, and the mobile phase comprised a mixture of phosphate buffer and acetonitrile. The separation of all compounds was achieved within 2 min. This method was successfully applied for stability evaluation of the developed formulations, which are now being used in the therapy of acid-related disorders in paediatric patients.


 Please wait while we load your content...
Please wait while we load your content...