Chiral micellar electrokinetic chromatographic separation for determination of l- and d-primary amines released from murine islets of Langerhans†
Abstract
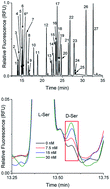
D-Amino acids have been located in various tissues including the endocrine portion of the pancreas, the islets of Langerhans. D-Serine (D-Ser) is of particular interest since it is an agonist for the ionotropic N-methyl-D-aspartate receptors. To examine the potential release of D-Ser and other D-amino acids from islets, a chiral micellar electrokinetic chromatography method was developed by derivatizing primary amines with 2,3-naphthalenedicarboxaldehyde and to achieve resolution of the enantiomers, two surfactants were used in the separation, sodium dodecyl sulfate and sodium deoxycholate. With the optimized conditions, 36 small molecule standards, including four internal standards, were evaluated. For the 17 compounds that were fully resolved, limits of detection were less than 10 nM. The resulting optimized separation method produced high efficiency peaks, with an average of 300 000 theoretical plates per peak and a peak capacity of 120. The method was used to examine the release of small molecules from groups of 50 murine islets of Langerhans. A peak was detected from islets incubated with 20 mM glucose that co-migrated with a D-Ser standard, although its level was below the quantifiable limit.
- This article is part of the themed collection: Analytical Methods Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...