Quantitative determination and validation of topiramate and its tablet formulation by 1H-NMR spectroscopy
Abstract
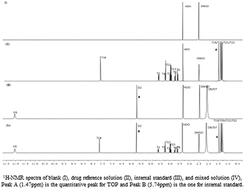
In this study, a relatively simple and precise novel quantitative nuclear magnetic resonance spectrometry method to determine topiramate in a tablet formulation was developed on a 400 MHz NMR instrument. DMSO-d6 as the NMR solvent was selected. The signals of the methyl proton of topiramate appeared as four independent singlet peaks (δ1.47 ppm, δ1.57 ppm, δ1.34 ppm, and δ1.29 ppm) with baseline separation. The δ1.47 ppm (singlet) peak was selected for quantification. The δ5.74 ppm (singlet) of 3,5-dimethylpyrazole (DMP) was selected as the internal standard. The development and validation of this method were carried out as per International Conference on Harmonization (ICH) guidelines. Linearity (R2 = 0.9992) was observed between 0.05 and 0.85 mg mL−1. The relative standard deviation for accuracy and precision was 2.0% or less. The limits of detection (LOD) and quantification (LOQ) were 0.04 and 0.16 mg mL−1, respectively. The robustness of the method was also investigated. The results showed that the method was accurate for the quantification of the topiramate tablet formulation, and was consistent with the method from the US Pharmacopoeia.


 Please wait while we load your content...
Please wait while we load your content...