A highly sensitive and selective fluorescent probe for nitroxyl based on a naphthalene derivative†
Abstract
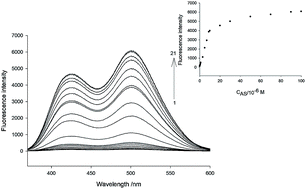
In this work, we developed a novel fluorescent probe 1 for quantitative detection of nitroxyl (HNO). Probe 1 consisted of a naphthalene backbone and 2-(diphenylphosphino)benzoate which was used as a nitroxyl recognition unit. The probe exhibited an intense fluorescence turn-on response to nitroxyl via aza-ylide formation and its subsequent Staudinger ligation to release compound 2. The probe can be applied to the quantification of nitroxyl with a linear range from 5.0 × 10−8 to 9.0 × 10−6 mol L−1. The detection limit of probe 1 toward nitroxyl was estimated to be 43 nM. Furthermore, probe 1 displayed a much higher selectivity for nitroxyl than other biologically relevant species. Importantly, owing to the high cell permeability and low cytotoxicity of probe 1, it was successfully applied to cell imaging of nitroxyl in living cells. The probe is expected to be a useful chemical tool for investigating the detailed functions and mechanisms of HNO in living systems.


 Please wait while we load your content...
Please wait while we load your content...