Direct bioelectrocatalysis by redox enzymes immobilized in electrostatically condensed oppositely charged polyelectrolyte electrode coatings†
Abstract
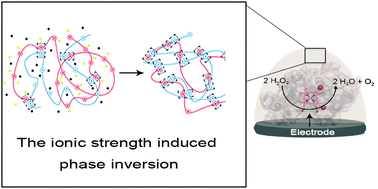
The immobilization of enzymes on an electrode surface is critical in preserving enzyme activity and providing a sufficient electron transfer pathway for bioelectrocatalysis. Here, we present a novel single-step, cross-linker free immobilization for direct bioelectrocatalysis using an ionic strength induced phase inversion of oppositely charged polyelectrolytes. Cationic poly-guanidinyl-propyl-methacrylate (pGPMA, PG) and anionic inorganic polyphosphate, sodium hexametaphosphate (P6) were used to make an electrostatically condensed phase (PGP6). A mixture of PGP6 and laccase (LAC) from Tramates versicolor or HRP (HRP) from Armoracia rusticana were deposited on the electrode surface and were submerged in DI water to form white porous electrode coatings. Each electrode showed a current generation corresponding to the respective substrates via direct bioelectrocatalysis.
- This article is part of the themed collection: Versatile Electrochemical Approaches


 Please wait while we load your content...
Please wait while we load your content...
