Matrix-assisted nanoelectrospray mass spectrometry for soft ionization of metal(i)–protein complexes†
Abstract
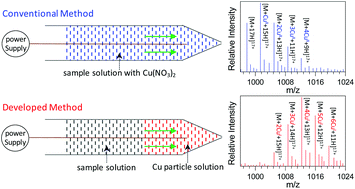
Metal ions play significant roles in biological processes, and investigation of metal–protein interactions provides a basis to understand the functions of metal ions in such systems. In the current study, a novel matrix-assisted nanoelectrospray ionization mass spectrometry (MANESI-MS) method was developed for investigating the interactions between metal ions (i.e., Cu+) and protein molecules (i.e., myoglobin) using Cu nanoparticles as the matrix. The results demonstrated that the present method not only was an efficient strategy for the generation of various complexes with monovalent metal ions, such as Cu+, in which no redox transitions between Cu+ and Cu2+ were observed, but also allowed a softer ionization of the generated Cu+–myoglobin complexes compared to that of myoglobin molecules with conventional nanoESI. Several parameters (i.e., the mixing mode of the myoglobin sample and Cu nanoparticle solution, size of the Cu particle, oxidation state of the Cu species, and acidity of the myoglobin solution) were found to be crucial in determining the ionization efficiency of the MANESI method. First loading a Cu nanoparticle solution into the electrospray tip followed by a myoglobin solution resulted in a favorable interaction between the generated Cu+ ions and myoglobin molecules, in which a smaller size of the Cu particle and a lower oxidation state of the metal species (Cu > Cu2O > CuO) gave a lower average charge state and hence a softer ionization of the resulting Cu+–myoglobin complexes, possibly due to the reduced denaturing effects of the Cu+ complex. The MANESI method has also been successfully used to ionize the complexes between Cu+ and other biological molecules such as cytochrome c and angiotension II, although an exception was found for lysozymes, which show an increase in the charge state. Analogous to the study with Cu, a variety of other metal nanoparticles (Ni, Fe, W, Ag, Al, Zn and Co) were explored to study their interactions with myoglobin, but only Zn and Co could produce monovalent metal ions (i.e., Zn+ and Co+) followed by a favorable interaction with myoglobin, and a soft ionization of the resulting complexes.


 Please wait while we load your content...
Please wait while we load your content...