Synthesis, application and molecular modeling study of ionic liquid functionalized lactobionic acid, 3-methyl-1-(3-sulfopropyl)-1H-imidazol-3-ium lactobionate, as a chiral selector in capillary electrophoresis
Abstract
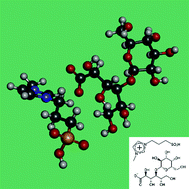
Recently, ionic liquids have been widely used for chiral separation. However, several papers have reported the use of ionic liquids as the sole chiral selector. In this work, a novel capillary electrophoresis method using the ionic liquid 3-methyl-1-(3-sulfopropyl)-1H-imidazol-3-ium lactobionate (MSI-LA) as a chiral selector was developed for the enantioseparation of propranolol, metoprolol, bisoprolol, esmolol, atenolol, sotalol, terbutaline and clenbuterol. Method optimization was conducted and the optimized method (40 mM borax buffer, v/v 30% methanol, pH 8.5, 160 mM MSI-LA; +10 kV voltage; capillary temperature, 15 °C; 50 mbar/5 s injection; detection wavelength, 230 nm) was successful for the baseline separation of the eight basic drugs. The LA (lactobionic acid) system was compared. The possible mechanisms of enantioseparation were investigated using molecular modeling. Both the cation and anion of MSI-LA provided interactions between the chiral selector and analytes, and finally enhanced chiral recognition capability compared with LA.


 Please wait while we load your content...
Please wait while we load your content...