An intramolecular charge transfer and excited state intramolecular proton transfer based fluorescent probe for highly selective detection and imaging of formaldehyde in living cells†
Abstract
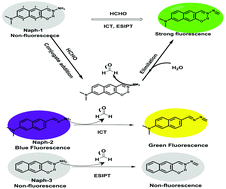
Formaldehyde (FA), as a reactive carbonyl species, is endogenously generated in various biological processes. Abnormal levels of FA could lead to various cellular dysfunction and pathological conditions. Here, we develop a new activatable fluorescent probe for highly selective visualization of FA in living cells. Our probe (Naph-1) is designed using a naphthalene derivative as the fluorophore and hydrazone as a recognition site for FA. Naph-1 is essentially nonemissive. After reacting with FA, the amine moiety is converted into a Schiff base with electron-withdrawing ability and the fluorescence is simultaneously turned on due to synergetic intramolecular charge transfer and favoured excited state intramolecular proton transfer effects. Naph-1 exhibits a large Stokes shift upon reaction with FA. Furthermore, it possesses high selectivity and superior sensitivity toward FA with an estimated limit of detection of 0.35 μM. Moreover, Naph-1 is also successfully applied to image both endogenous and exogenous formaldehyde in living cells. These features demonstrate that Naph-1 holds great potential in the detection and imaging of formaldehyde in biological systems.

 Please wait while we load your content...
Please wait while we load your content...