Pyridine-hydrazone-controlled cyanide detection in aqueous media and solid-state: tuning the excited-state intramolecular proton transfer (ESIPT) fluorescence modulated by intramolecular NH⋯Br hydrogen bonding†
Abstract
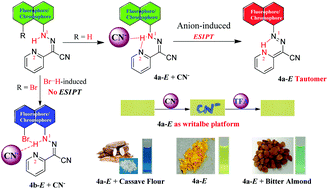
A new efficient pyridine-hydrazone-substituted naphthalimide receptor 4a-E has been synthesized as a selective colorimetric and fluorescent chemosensor for cyanide sensing in aqueous environments through a unique excited-state intramolecular proton transfer (ESIPT) mechanism. The addition of a Br group to the fluorophore skeleton at the ortho-position of hydrazone generates reference compounds (4b-E and 4c-Z). Interestingly, the potential intramolecular NH⋯Br hydrogen bonding might compete with the anion-induced intermolecular NH⋯A− hydrogen bonding, resulting in dramatic ESIPT suppression. The high emission of probe 4a-E and other control probes in solid-state is also investigated. Moreover, probe 4a-E preloaded on test papers, upon cyanide treatment, shows obvious changes in color which demonstrates that 4a-E is a writable platform. More importantly, it exhibits great potential application for the detection of cyanide in food materials and excellent performance in real-world water samples.


 Please wait while we load your content...
Please wait while we load your content...