A lysosome specific theranostic NO donor inhibits cancer cells by stimuli responsive molecular self-decomposition with an on-demand fluorescence pattern†
Abstract
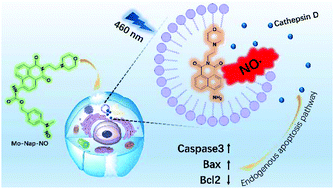
The anticancer mechanism of NO is difficult to study owing to its short lifetime and high reactivity. Thus, a theranostic anticancer NO donor assembled with NO on-demand release abilities, accurate lysosome location capabilities and signal feedback behavior was developed. Profiting from the theranostic properties, the specific mechanism was comprehensively studied. Spectral and cell imaging studies revealed that the as prepared NO donors could release NO in solution or within cancer cells. Fluorescence co-dyeing experiments demonstrated that Mo-Nap-NO entered lysosomes specifically and disrupted them after being triggered by light. Upon irradiation with 460 nm visible light, both the donors demonstrated considerable in vitro anticancer effects. A further mechanistic study showed that after entering the lysosome and being triggered by 460 nm irradiation, NO ruptured the lysosome, resulting in the release of cathepsin D into the cytosol, which activated the caspase3 mediated apoptosis pathway.


 Please wait while we load your content...
Please wait while we load your content...