Efficient enantiorecognition of amino acids under a stimuli-responsive system: synthesis, characterization and application of electroactive rotaxane†
Abstract
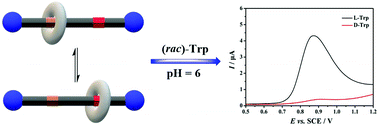
In this study, an electroactive rotaxane, (S,S)-crown-3, consisting of a polymeric chiral ionic liquid as a flexible axle and 18-crown-6 as the wheel, was designed and synthesized. It is worth noting that a stimuli-responsive system was developed, in which the wheel could switch its location between the chiral carbamido group and ionic pair of the ionic polymers under an external force. Next, (S,S)-crown-3 was employed as a modification on the surface of glassy electrode. In contrast to previous study, the developed probe presented a clear discrimination of an electrochemical signal in the absence of Cu(II). Under the external force (different pH values), L-isomers of amino acids (tryptophan, tyrosine, and cysteine) could form stable host–guest interactions with the chiral carbamido group, producing higher peak currents than the D-isomers. Compared to the absence of the crown, (S,S)-crown-3 showed much better recognition efficiency. The value of IL/ID for tryptophan could reach 39.8. In brief, the present study describes a powerful method for the synthesis of an electroactive rotaxane with great enantiorecognition capability.


 Please wait while we load your content...
Please wait while we load your content...