Enantioseparation of amino alcohol drugs by nonaqueous capillary electrophoresis with a maltobionic acid-based ionic liquid as the chiral selector†
Abstract
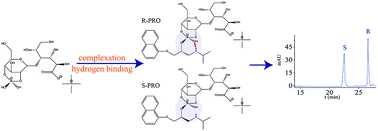
This study deals with the nonaqueous capillary electrophoretic enantioseparation of twenty-two amino alcohol drugs with a maltobionic acid (MA)-based ionic liquid (tetramethylammonium maltobionic acid, TMA-MA) as the novel chiral selector. In consideration of the poor solubility of MA in organic solvents, we managed to transform MA into ionic liquids (ILs) for the first time. Interestingly, this chiral selector exhibited powerful enantioselectivity towards the model analytes in company with boric acid. Systematical experiments were carried out to investigate the influence of concentration of TMA-MA, boric acid and tris (hydroxymethyl) aminomethane (Tris) as well as applied voltage on the enantioseparation. A great majority of enantiomers (except labetalol) were baseline separated under the optimized conditions and the effect of the molecular structure of amino alcohol drugs on the chiral separation was discussed. In addition, electrophoretic experiments, nuclear magnetic resonance (NMR), mass spectrometry (MS) and molecular modeling with the Gaussian program were employed to demonstrate the mechanism of chiral recognition. Based on the formation of an ionic liquid-boric acid-analyte complex, hydrogen binding was mainly responsible for enantioseparation.


 Please wait while we load your content...
Please wait while we load your content...