Estimation of bisulfate in edible plant foods, dog urine, and drugs: picomolar level detection and bio-imaging in living organisms†
Abstract
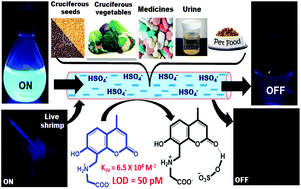
In order to explore the properties of any species in solution, the actual, i.e. equilibrium concentration of the free species should be taken into account. Researchers have not paid attention to the deprotonation equilibrium between HSO4− and SO42− while probing bisulfate ion. In this study, we have addressed this concern and developed two zwitterions, CG (coumarin-integrated glycine) and CA (coumarin-integrated alanine), for the selective detection of HSO4− at a picomolar level (50 to 325 pM) with very high binding affinity (∼108 M−1) in pure water at physiological pH. The principle of HSO4− recognition was established via UV-vis and fluorescence techniques. DFT calculations suggested that the H-bonding interactions between the probes and HSO4− are the driving force for this unforeseen selectivity. The membrane penetration ability and nontoxicity of CG/CA enable them to function as staining agents in living brine shrimps and bacteria. The use of these probes for the estimation of HSO4− in various day-to-day edible foods and drugs along with urine samples is unprecedented. The significance and novelty of this study lies in the application and development of assays for estimating bisulfate in several real-world samples that are predominantly aqueous in nature, which are the first of their kind.


 Please wait while we load your content...
Please wait while we load your content...