In situ study of RSK2 kinase activity in a single living cell by combining single molecule spectroscopy with activity-based probes†
Abstract
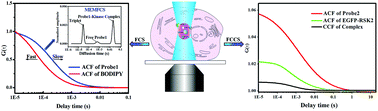
Protein phosphorylation is a very important regulatory mechanism in a majority of biological processes, and the determination of protein kinase activity plays a key role in the pathological study and drug development of kinase-related diseases. However, it is very challenging to in situ study endogenous protein kinase activity in a single living cell due to the shortage of in vivo efficient methods. Here, we propose a new strategy for direct determination of protein kinase activity in a single living cell by combining single molecule fluorescence correlation spectroscopy (FCS) with activity-based probes (ABPs). Ribosomal S6 kinase-2 (RSK2) was used as a model, and the ABPs were synthesized on the basis of RSK2 inhibitor FMK to specially label active RSK2 in living cells. Conventional FCS and MEMFCS (maximum entropy method) single molecule techniques were used to in situ determine RSK2 activity in living cells based on the difference in molecular weight between free probes and probe–RSK2 complexes. Furthermore, wild-type and mutated RSK2 were fused with enhanced green fluorescent protein (EGFP) using lentivirus infection, and fluorescence cross-correlation spectroscopy (FCCS) was used to verify the selective binding of ABPs to RSK2-EGFP fusion protein in living cells. Finally, FCS with ABPs was applied for in situ monitoring of the activation of endogenous RSK2 in the stimulation of serum, epidermal growth factor, kinase inhibitors and ultraviolet irradiation; we observed that endogenous RSK2 showed different behaviors in the cytoplasm and the nucleus in some stimulation. Our results document that FCS with ABPs is a very promising method for studying endogenous protein kinases in living cells.


 Please wait while we load your content...
Please wait while we load your content...