Facile method based on 19F-NMR for the determination of hydroxyl value and molecular weight of hydroxyl terminated polymers†
Abstract
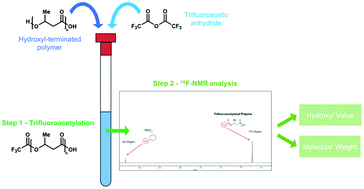
A new, facile and low-sample consuming technique for the determination of hydroxyl values (OHVs) in poly(hydroxyalkanoate)s (PHAs) is herein presented. After a fast, uncatalyzed and non-destructive trifluoroacetylation of all PHA hydroxyl groups, OHVs are calculated through 19F-NMR measurements, comparing integral values of the only two signals in the spectra: the fluorinated hydroxyl groups and the internal standard (trifluorotoluene) signals. Furthermore, the combined ex situ functionalization with a simple workup of the fluorinated polymer allowed to obtain spectra with an improved signal/noise ratio and short acquisition time if compared to 1H-NMR-based methods. The results obtained by 19F-NMR measurements are then validated by the established standard method based on acid/base titration and the 1H-NMR-based method. The proposed approach shows to be valuable not only for low molecular weight PHAs, but also for high molecular weight samples, which typically cannot be analysed by conventional 1H-NMR-based methods due to the very low concentration of hydroxyl terminal groups. Thanks to the observed high reliability, the method was also successfully applied to evaluate the OHVs of various commercial hydroxyl terminated polymers with different molecular weights in order to further show its wide applicability.


 Please wait while we load your content...
Please wait while we load your content...