Reliable method for the detection of horseradish peroxidase activity and enzyme kinetics†
Abstract
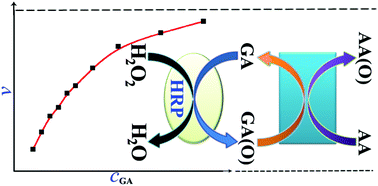
Enzyme-catalyzed reactions are complicated and their kinetics depend on various chemical and physical factors. In a simple enzyme-catalyzed reaction, the enzyme kinetics often involve two or more substrates. However, this complexity is often ignored when studying enzyme kinetics or determining enzyme activity. Such an example is horseradish peroxidase (HRP), whose activity and kinetics in the reduction of H2O2 are usually detected and studied using spectroanalysis, with guaiacol (GA) as the hydrogen donor. In this process, the concentrations of two substrates, GA and H2O2, both change, which makes the practical detection, based on determination of the GA oxydate, GA(O), totally wrong. In this study, we introduce a new electrochemical method for detecting the specific activity (SA) and studying the enzyme kinetics of HRP. This electrochemical method was used to directly detect one substrate (H2O2) while the concentration of the other substrate (GA) was kept constant by adding ascorbic acid to the system to reduce GA(O) and regenerate GA. For the first time, this HRP-catalyzed reaction, including the mechanism and kinetics, was investigated precisely using a simple electrochemical method. The maximum SA and reaction rate constant k1 were reliably detected and calculated. The proposed method indicated that the SA of commercially available HRP (300 U mg−1 detected by spectroanalysis) was 1228.8 U mg−1 at a GA concentration of 4.5 mM, and up to 2049.9 U mg−1 as the GA concentration tended toward infinity. Our results suggest that reported methods for detecting enzyme activity and/or kinetics should be re-examined according to the catalytic mechanism of the enzyme.


 Please wait while we load your content...
Please wait while we load your content...