From inorganic precipitation to organic aggregation: solubility product constant-mediated specific metal-ion lighting-up using a triazolium iodide organic fluorescence tag†
Abstract
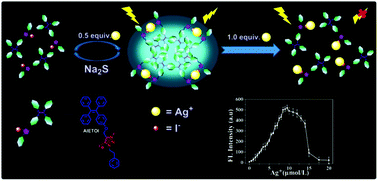
Innovation in sensing strategies is a continual goal pursued by analytical chemists. This work reports for the first time a solubility product constant (Ksp)-mediated specific aggregation phenomenon, which is well applicable for selective metal-ion sensing, where the conventional inorganic ion binding is replaced by the anion-induced aggregation of organic molecules. A fluorescent triazolium iodide with aggregation-induced emission (AIETOI) was synthesized via click decorating a triazole onto the tetraphenylethylene backbone followed with cationization of the triazole ring. The novel probe afforded excellent specific lighting-up capability of mercury or silver ions at the ppm level followed by fluorescence quenching with excess Hg2+ or Ag+. The extremely low Ksp of HgI2 or AgI was proven to be responsible for the specific recognition ability. Hg2+/Ag+ can coordinate with the semi-dissociated iodide causing the aggregation and fluorescence lighting-up of the AIETOI, and the subsequent fluorescence quenching can be ascribed to the “peeling” of AIETOI molecules from the aggregates. The sensing process was relatively inert to pH, ionic strength and other ion interferences, providing an interesting perspective for sensing platform design.


 Please wait while we load your content...
Please wait while we load your content...