Rational design of a boron-dipyrromethene-based fluorescent probe for detecting Pd2+ sensitively and selectively in aqueous media†
Abstract
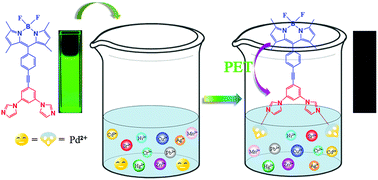
A novel fluorescent probe for Pd2+ based on the BODIPY fluorophore exploiting the PET (Photoinduced Electron Transfer) mechanism was designed and successfully synthesized. The fluorescent probe 1 was prepared by introducing m-bisimidazolylbenzene which was connected by phenyl acetylene to the BODIPY dye at the meso position. It exhibited a rapid response and high sensitivity and selectivity toward Pd2+. Probe 1 presented a rapid quenched fluorescence response in aqueous buffer media (pH 5.5) and the detection limit estimated from the titration results was 2.9 × 10−7 M. Meanwhile, other common metal ions did not interfere with the recognition process. The DFT calculation proved that coordination of bisimidazole ligands with Pd2+ effectively decreases the LUMO energy of m-bisimidazolylbenzene which was located between the HOMO and LUMO energies of the BODIPY dye leading to fluorescence quenching via the d-PET mechanism.
- This article is part of the themed collection: In celebration of Chinese New Year 2020


 Please wait while we load your content...
Please wait while we load your content...