A nontoxic reversible thermochromic binary system via π–π stacking of sulfonephthaleins†
Abstract
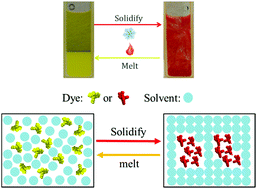
The mechanisms of thermochromic materials are mostly based on Bragg reflection, surface plasmon absorption, molecular structure changes, conformational stretching or aggregation. Here, a simple class of dye–solvent binary systems with clear and reversible thermochromism around ambient temperatures is reported with an alternative mechanism. This system comprises commercially available sulfonephthaleins dissolved in liquid linear chain esters, acids or alcohols, which demonstrate colour changes at different temperatures when the solvents transition from solid to liquid states. The colour changing mechanism is based on π–π stacked aggregation or disaggregation of the sulfonephthalein dyes. Cell toxicity tests confirmed that the sulfonephthalein dyes are less toxic to NIH-3T3 cells than cyanidin chloride, which is the only reported nontoxic food dye affording thermochromism, while this is based on an alternative mechanism. With the choice of a nontoxic solvent, this binary system can form a nontoxic thermochromic material.


 Please wait while we load your content...
Please wait while we load your content...