Controlling intermolecular redox-doping of naphthalene diimides†
Abstract
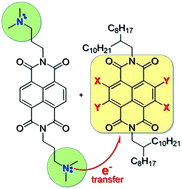
Naphthalene diimide (NDI) with tertiary amine side chains is used to n-dope a series of NDI derivatives of varying energy levels. We demonstrate a photoinduced, intermolecular redox-doping process in which a dimethylpropyl amine side chain attached to one NDI reduces another NDI derivative to form radical anions. The influence of the aromatic core substituents on energy levels, doping efficacy and radical anion stability is studied by cyclic voltammetry, UV-Vis and electron paramagnetic resonance (EPR) spectroscopy. In general, the HOMO energy level of the NDI is responsible for the doping process and the LUMO for air stability of the resulting radical anion. The most electron deficient NDI derivative having two cyano substituents displays the highest doping yield and yields air stable radical anions for both light- and thermally-induced doping. Thermal doping is further accompanied by morphologic changes that stabilize radical anions in air.


 Please wait while we load your content...
Please wait while we load your content...