Benzoselenadiazole and benzotriazole directed electrophilic C–H borylation of conjugated donor–acceptor materials†
Abstract
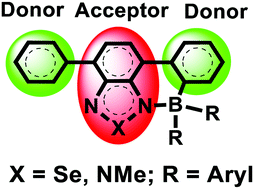
Benzoselenadiazole and benzotriazole directed electrophilic borylation using BCl3 results in the C–H functionalization of an adjacent aromatic unit and produces fused boracycles. Subsequent arylation at boron afforded air and moisture stable products displaying large bathochromic shifts and significantly reduced LUMO energy levels. OLEDs fabricated containing borylated benzoselenadiazole derivatives showed emission centered at 723 nm in the near infra-red region of the spectrum.


 Please wait while we load your content...
Please wait while we load your content...