MnFe0.5Ru0.5O3: an above-room-temperature antiferromagnetic semiconductor†
Abstract
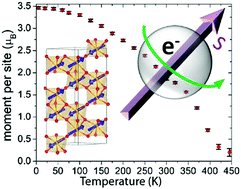
A transition-metal-only MnFe0.5Ru0.5O3 polycrystalline oxide was prepared by a reaction of starting materials MnO, MnO2, Fe2O3, RuO2 at 6 GPa and 1873 K for 30 minutes. A combination of X-ray and neutron powder diffraction refinements indicated that MnFe0.5Ru0.5O3 adopts the corundum (α-Fe2O3) structure type with space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, in which all metal ions are disordered. The centrosymmetric nature of the MnFe0.5Ru0.5O3 structure is corroborated by transmission electron microscopy, lack of optical second harmonic generation, X-ray absorption near edge spectroscopy, and Mössbauer spectroscopy. X-ray absorption near edge spectroscopy of MnFe0.5Ru0.5O3 showed the oxidation states of Mn, Fe, and Ru to be 2+/3+, 3+, and ∼4+, respectively. Resistivity measurements revealed that MnFe0.5Ru0.5O3 is a semiconductor. Magnetic measurements and magnetic structure refinements indicated that MnFe0.5Ru0.5O3 orders antiferromagnetically around 400 K, with magnetic moments slightly canted away from the c axis. 57Fe Mössbauer confirmed the magnetic ordering and Fe3+ (S = 5/2) magnetic hyperfine splitting. First principles calculations are provided to understand the electronic structure more thoroughly. A comparison of synthesis and properties of MnFe0.5Ru0.5O3 and related corundum Mn2BB′O6 derivatives is discussed.
c, in which all metal ions are disordered. The centrosymmetric nature of the MnFe0.5Ru0.5O3 structure is corroborated by transmission electron microscopy, lack of optical second harmonic generation, X-ray absorption near edge spectroscopy, and Mössbauer spectroscopy. X-ray absorption near edge spectroscopy of MnFe0.5Ru0.5O3 showed the oxidation states of Mn, Fe, and Ru to be 2+/3+, 3+, and ∼4+, respectively. Resistivity measurements revealed that MnFe0.5Ru0.5O3 is a semiconductor. Magnetic measurements and magnetic structure refinements indicated that MnFe0.5Ru0.5O3 orders antiferromagnetically around 400 K, with magnetic moments slightly canted away from the c axis. 57Fe Mössbauer confirmed the magnetic ordering and Fe3+ (S = 5/2) magnetic hyperfine splitting. First principles calculations are provided to understand the electronic structure more thoroughly. A comparison of synthesis and properties of MnFe0.5Ru0.5O3 and related corundum Mn2BB′O6 derivatives is discussed.


 Please wait while we load your content...
Please wait while we load your content...