Amine additive reactions induced by the soft Lewis acidity of Pb2+ in halide perovskites. Part I: evidence for Pb–alkylamide formation†
Abstract
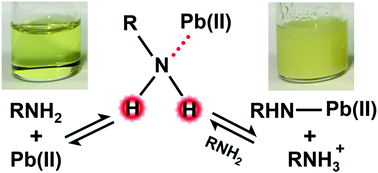
Metal halide perovskite materials (ABX3) employed in optoelectronic devices have rapidly achieved high efficiencies. Their performance can be partially attributed to intrinsic defects being electronically benign. Despite the inertness of defects within the bulk, such as ion vacancies, solution processing additives and surface passivation have been shown to enhance material properties. Thus, detailed knowledge of additive chemistry is desirous to elucidate passivation mechanisms and facilitate further improvements. In particular, the soft Lewis acid nature of Pb2+ has been overlooked heretofore as a key feature of amine-lead chemistry in perovskite preparations. In Part I of this study, we demonstrate that acid–base reactions between PbI2 and aliphatic amines yield alkylammonium and Pb–alkylamide species. The alkylammonium and alkylamide products are capable of occupying A-site cation and anion vacancies, respectively. Subsequent incorporation of these impurities at perovskite crystal surfaces or formation of non-perovskite phase inclusions likely influence the perovskite layer morphology and properties which are investigated in Part II. These results reveal a new mechanism by which optoelectronic properties of perovskites can be chemically modified. Additionally, the general concept of acidification of organic molecules solvated to Pb2+ promoting unexpected reactions is applied to explain amine reactions with other additives, such as formic acid. Understanding acid–base reactions induced by PbI2 complexing to protic molecules is useful to comprehend and predict defect/impurity chemistry in a wide variety of perovskite precursor formulations.
- This article is part of the themed collections: Electronic Properties and Characterisation of Perovskites and 2019 Journal of Materials Chemistry C Most Popular Articles


 Please wait while we load your content...
Please wait while we load your content...