Interfacial interaction of anesthetic lidocaine and mesoporous silica nanoparticles in aqueous solutions and its release properties
Abstract
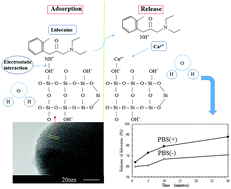
Lidocaine has been used as a local anesthetic by injection. The controlled release of lidocaine loaded into nanospheres is necessary to reduce the onset time of the anesthetic effect or increase the anesthetic analgesia duration. In this study, mesoporous silica nanoparticles (MSNs) with a large specific surface area were prepared by a sol–gel method, and the interfacial interaction between MSNs and lidocaine positively charged in aqueous solutions at different concentrations was investigated by adsorption tests, Fourier-transformed infrared spectroscopy, thermogravimetry–differential thermal analysis, and Brunauer–Emmett–Teller (BET) measurements. The electrostatic interaction between Si–OH on MSNs and lidocaine-NH+ was of importance for the adsorption phenomenon in aqueous solutions, indicating the monolayer adsorption of lidocaine. BET measurements also supported the decrease of pore volumes, and the hysteresis loop of the isotherm curve was not closed since the condensation of lidocaine in the mesopores formed micropores of less than 1.5 nm in size. The release profiles in phosphate buffered saline containing calcium and magnesium ions showed a rapid and higher release of lidocaine compared with that in phosphate buffered saline without divalent cations. The released lidocaine concentrations were sufficient for the expression of the anesthetic effect in dental anesthesia.


 Please wait while we load your content...
Please wait while we load your content...