Are type 316L stainless steel coin cells stable in nonaqueous carbonate solutions containing NaPF6 or KPF6 salt?†
Abstract
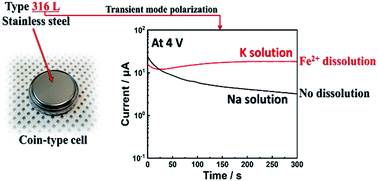
In this study, the suitability of type 316L stainless steel as coin cell components for nonaqueous sodium- and potassium-ion batteries is investigated. The passivation of type 316L stainless steel in nonaqueous carbonate solutions containing NaPF6 or KPF6 salt is examined using electrochemical dynamic polarization, transient polarization with in situ surface scratching, surface analysis using time-of-flight secondary ion mass spectroscopy, and X-ray photoelectron spectroscopy. The passivation layer formed on the surface of type 316L stainless steel after polarization is composed of metal fluorides (CrF3 and FeF3) and metal oxides (Cr2O3 and Fe2O3). In the presence of NaPF6, the type 316L stainless steel is well passivated without corrosion; however, selective dissolution of Fe2+ from the passive layer of the type 316L stainless steel occurs at a polarization potential of 4 V vs. K+/K, which could affect the reliability of experimental results when this material is used as coin cell components.


 Please wait while we load your content...
Please wait while we load your content...