Itinerant ferromagnetic half metallic cobalt–iron couples: promising bifunctional electrocatalysts for ORR and OER†
Abstract
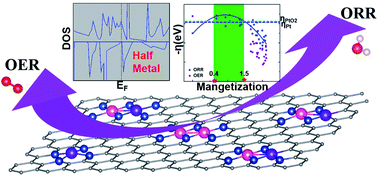
Spin polarization has been recently recognized as a critical factor that affects the catalytic behavior of electrocatalysts. Half metals, with an exotic quantum state of 100% spin-polarized conduction electrons, and the spin associated atomic magnetization could show great potential in catalyst applications, however the corresponding research is insufficient. In this work, a prototype study of (Co and/or Fe)–Nx (x = 1–6) embedded configurations (FeCoNx–gra) is conducted within density functional theory (DFT) via spin-polarized calculation. The results indicate that an itinerant ferromagnetic half metal can be recognized as a promising candidate for a bifunctional electrocatalyst for both oxygen reduction reaction (ORR) and oxygen evolution reaction (OER). Specifically, the ferromagnetically coupled spin-polarized electrons in a half metal tend to attract oxygen molecules so as to stimulate ORR, while the formation of the O–O bond in OER requires spin conservation. The delocalized spin character (metallic spin of a half metal) guarantees the moderate binding strength of main reaction intermediates, i.e., *O2, *O, *OH, and *OOH, which can be measured by the atomic spin moment in the reaction center (0.4–1.5 μB for Fe in this study). This study identifies a new specific link between the electron structure and catalytic activities which has predictive power for catalyst design.


 Please wait while we load your content...
Please wait while we load your content...