Mitigating concentration polarization for highly reversible plating/stripping electrochemistry: Li versus Na†
Abstract
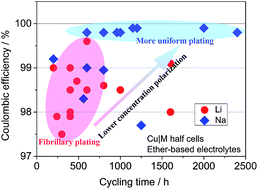
Lithium and sodium metal anodes are plagued with non-uniform plating and vigorous chemical reactivity despite their high theoretical capacities and low working potentials. However, latest reports show that the coulombic efficiencies (CEs) and cycling lifespan of Na metal anodes are evidently superior to those of Li. Herein, we compare the plating/stripping processes of Li and Na metals and reveal that the lower CEs of Li are related to the fibrillary plating behavior originating from severer concentration polarization. It is found that fresh solid electrolyte interphases (SEIs) are continuously formed in association with the fibrillary growth of Li, thus reducing the CEs and consuming the electrolyte with cycling; however, the SEI reformation on Na is greatly suppressed as Na plating proceeds uniformly beneath the previously formed SEI, resulting in higher CEs. Consequently, Li exhibits a much more pronounced relationship of cycle life with the amount of the electrolyte than Na. Li can be cycled on bare Cu 550 times with 110 μL electrolyte. Furthermore, it is shown that the SEI on Li metal has a higher ion transport barrier compared with that on Na, and the concentration polarization at the electrode-SEI interface is responsible for the fibrillary plating of Li. This is proved by the inter-conversion in the geometry of Li and Na deposits when the concentration polarization is weakened for Li by increasing temperature and reinforced for Na by decreasing the sodium salt concentration. The CE of Li could be improved to 98.7% at 55 °C over 250 cycles. The mechanistic study in this work provides a suggestion to evaluate the reversibility of Li metal anodes more reasonably and a guidance to suppress concentration polarization and fibrillary growth, which may lead to highly reversible plating/stripping electrochemistry of Li and other metal anodes.


 Please wait while we load your content...
Please wait while we load your content...