Hexaazatriphenylene-based polymer cathode for fast and stable lithium-, sodium- and potassium-ion batteries†
Abstract
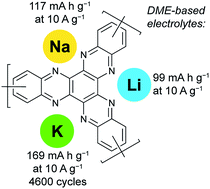
Organic redox-active compounds represent a promising family of materials for metal-ion batteries. They can be produced from renewable resources and contain no toxic or expensive heavy metals. Moreover, they are much less specific to the nature of the mobile ion, such as Li+, Na+ or K+, which facilitates the development of cheaper alternatives to the currently dominating lithium-ion battery technology. Here we report stable and ultrafast lithium-, sodium- and potassium-ion batteries (LIBs, SIBs and PIBs) comprising a polymer cathode based on hexaazatriphenylene, which is synthesized from 3,3′-diaminobenzidine and triquinoyl. Using LIBs as a model system, it is shown that the application of dimethoxyethane (DME) as the electrolyte solvent is crucial for achieving a superior performance. Using a DME-based electrolyte, a specific capacity of 169 mA h g−1 is reached for PIBs at an impressive current density of 10 A g−1 (charging/discharging in ca. one minute) after 4600 cycles. At a lower current density of 50 mA g−1, the capacity of PIBs approaches 245 mA h g−1. The performance of the designed polymer cathode is among the best ever reported for K-ion battery materials in terms of specific capacity, energy and power density. The polymer-based devices demonstrated record cycling stability, outperforming all non-aqueous potassium-ion batteries reported to date.


 Please wait while we load your content...
Please wait while we load your content...