Rechargeable aqueous electrolyte batteries: from univalent to multivalent cation chemistry
Abstract
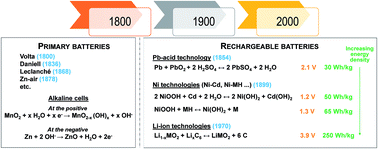
Water based electrolytes enable very high ionic conductivity, and are particularly attractive for high power density batteries. The main advantages of water-based electrolytes are their lower cost and non-flammability, while their principal disadvantage is the limited thermodynamic electrochemical window of water. Yet, the latter is currently being challenged, through the use of highly concentrated electrolytes (“water in salt concept”). Strong research focus is currently placed on rechargeable M-ion batteries (M = Li, Na) mimicking the organic Li-ion or Na-ion batteries, which will despite falling shorter in energy density exhibit cost advantages. Moreover, they should be expected to deliver very attractive power densities. The main challenge at this stage is the development of new negative electrodes able to operate at lower potentials. A more challenging topic is divalent ion concepts (M = Zn) using a Zn metal anode which could, in principle, deliver higher energy density, but for which issues still remain related to (i) developing appropriate positive electrode materials for reversible Zn ion insertion and (ii) side reactions involving mostly H+ or OH− species, which are not yet mastered.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...