Spatially confining and chemically bonding amorphous red phosphorus in the nitrogen doped porous carbon tubes leading to superior sodium storage performance†
Abstract
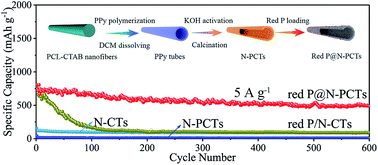
Red phosphorus has been widely studied as the most promising anode material for sodium ion batteries (NIBs) with an ultrahigh theoretical capacity of 2596 mA h g−1. However, its poor intrinsic electronic conductivity and huge volume change during the continuous sodiation/desodiation process lead to sluggish sodium ion diffusion kinetics and severe pulverization of the electrode material, resulting in severe capacity decay. In this study, a rationally designed P/C anode material of nitrogen doped porous carbon tubes (N-PCTs) with tiny red P embedded into the pores is successfully fabricated. The formed P–C chemical bonds could facilitate robust and intimate contact between red P and the carbon matrix and the robust structural integrity is confirmed by our in situ transmission electron microscopy (TEM) study of the sodiation/desodiation cycle. Benefiting from the synergistic effect of the physical confinement of the pores in the carbon matrix and the chemical adsorption due to the P–C chemical bonding, superior long-term cycling stability (1157 mA h g−1 at 2 A g−1 after 100 cycles) as well as excellent rate capability (572 mA h g−1 at 10 A g−1) could be obtained.


 Please wait while we load your content...
Please wait while we load your content...