Insights into the water adsorption mechanism in the chemically stable zirconium-based MOF DUT-67 – a prospective material for adsorption-driven heat transformations†
Abstract
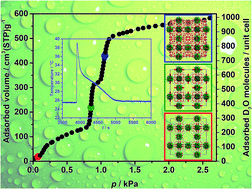
A chemically and thermally stable MOF with composition Zr6O4(OH)4(tdc)4(CH3COO)4 (tdc, 2,5-thiophenedicarboxylate), also known as DUT-67(Zr), was synthesised at the multigram scale using a green synthesis protocol as a potential material for adsorption heat pumps. A series of vapour physisorption experiments at 298 K identified water as the most promising working fluid, showing the desired S-shaped reversible physisorption isotherms with adsorption steps within the desired relative pressure range of p/p0 = 0.1–0.4. An enhanced long-term chemical stability of the MOF was proved in liquid water and mineral acid and thermal stability was confirmed in temperature dependent PXRD experiments. Stable performance of the material under working conditions was confirmed in 20 adsorption/desorption cycles under conditions typical for an adsorption pump. The mechanism of water adsorption was further studied by neutron powder diffraction, suggesting that the preferable adsorption sites for water are near the μ3-O and μ3-OH groups of the Zr6O8 cluster and the triangular window of the octahedral micropore, and the order of pore filling starts from the smallest pore, progressing to the middle and largest pore.


 Please wait while we load your content...
Please wait while we load your content...